Magnus provides key elements ideal for bone graft formation:
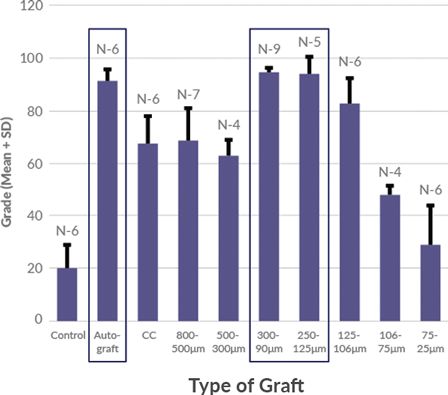
Magnus bone matrix provides a natural, osteoconductive bone scaffold composed of demineralized cortical and cancellous bone. The optimized microparticulate bone scaffold size range of 100-300 μm has been shown to induce simultaneous activity of osteoclasts and osteoblasts, demonstrating rapid healing of bone defects.
100-300 μm optimized particle size for bone regeneration has been shown to support direct ossification, with defect healing rates comparable to autograft.

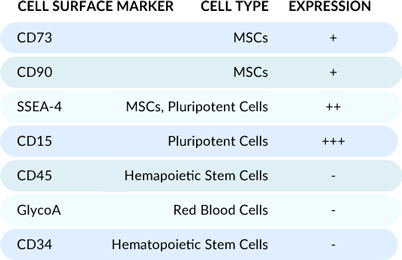
Flow cytometry analysis of the cells post-thaw reveals a unique cell population optimal for osteogenic supplementation, evidenced by high expression of MSC and pluripotent cell markers.

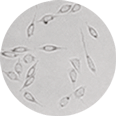
DMSO-free cryoprotectant coats the cells to prevent crystalline damage.

Magnus Representative Sample
2.5% DMSO Media Sample
Industry standard DMSO penetrates and dehydrates the cell from within to prevent crystal formation. At room temperature, DMSO-based cryoprotectants raise concerns about cytotoxicity and negative effects on cell differentiation.
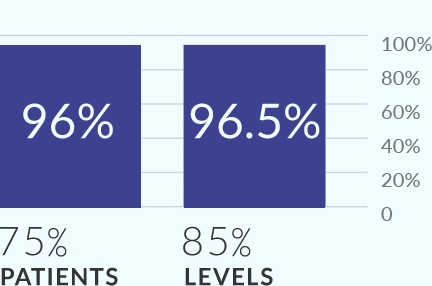
MIS-TLIF study demonstrated 96% fusion at 12 months.
TLIF FUSION with Allograft. 
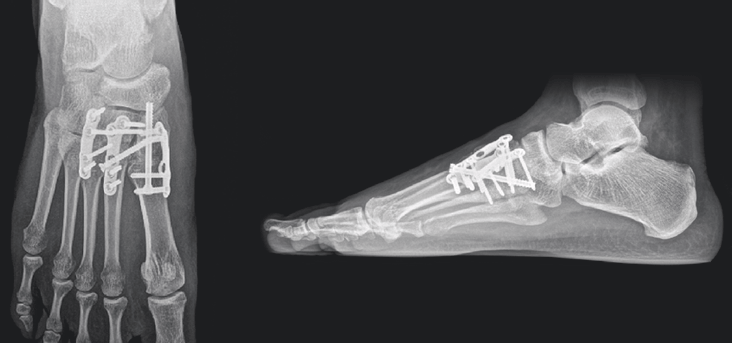
12-week post-operative X-rays show complete osseous consolidation of the arthrodesis sites and no pain or swelling with palpation or manipulation of the surgical sites.
Cortical shavings, crushed cancellous chips, and demineralized cortical bone microparticulate scaffold blend with bone gel mixture
Hydrophobic properties make it more resistant to lavage.
