
Lumbar fusion rates can range from 65%–90% when autogenous bone is harvested and used to fill voids in spine surgery.1,2 For many years, Iliac Crest Bone Graft (ICBG) has been the “gold standard.” However, surgeons are less inclined to use this technique due to donor site morbidity and the potential risk of a second surgical site complication. 2 For these reasons, surgeons have begun to explore other bone grafting options which have resulted in varying degrees of success.

The Fibrinet System was designed for the safe and rapid preparation of Platelet-Rich Fibrin Membrane (PRFM) and Platelet-Rich Fibrin Gel (PRFG) from a small sample of blood at the patient point of care.

Draw patient’s blood into the blood collection tubes and spin in a centrifuge for six minutes. After centrifugation, the concentrated platelets and plasma are separated from the other blood components.
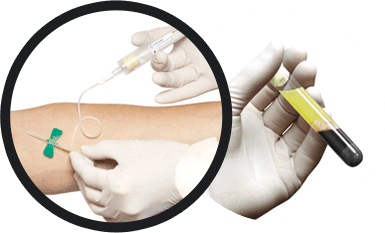

Resuspend platelets into the plasma; then transfer required volume into the membrane vial and spin in centrifuge for twenty-five minutes. After centrifugation, the membranes are ready to be delivered to the sterile field.
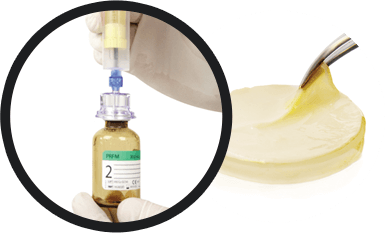

Using the remaining tube from first spin, mix concentrated platelets and plasma with bone grafting material manually or via centrifugation for desired handling characteristics.

The FIBRINET® System is an advanced alternative for lumbar fusion surgery as physicians evaluate biologic solutions to improve patient outcomes. The Fibrinet system represents an easy and efficient method to harness and concentrate the patient’s own platelets and associated growth factors into a Platelet-Rich Fibrin Membrane (PRFM) graft. In addition, Platelet-Rich Fibrin Gel is produced and mixed with fresh cancellous bone elements from the patient and bone grafting material, as defined by the surgeon, for improved handling characteristics. Two PRFM grafts and the Surgeon Defined Graft have been used successfully in a variety of spine applications and have demonstrated 100% radiographic fusion at 12 months in two separate studies.
Two separate studies have evaluated the use of the Fibrinet System in lumbar fusion surgery.3,4 Both studies showed a statistically significant decrease in the patients’ mean Oswestry Disability Index (ODI) scores and both demonstrated 100% radiographic fusion at 12 months. In one of the studies, the author concluded that the implantation of the PRFM intraoperatively may be responsible for the successful fusion mass seen radiographically.4 For each study, all surgeries were performed by a single surgeon at a single institution, and radiographic evaluations were done by an independent radiologist.

Concentrated platelets and their associated growth factors without thrombin.
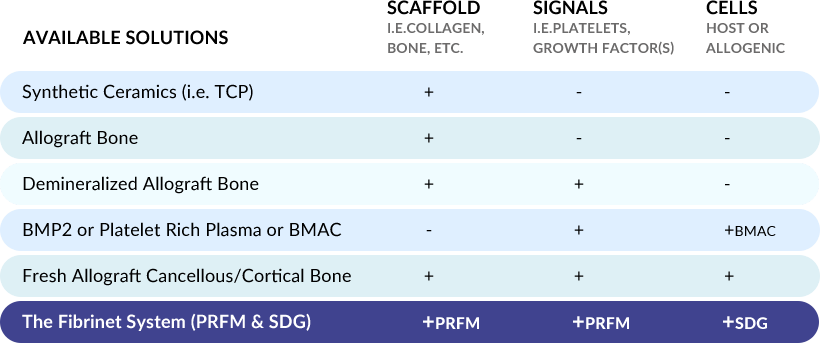
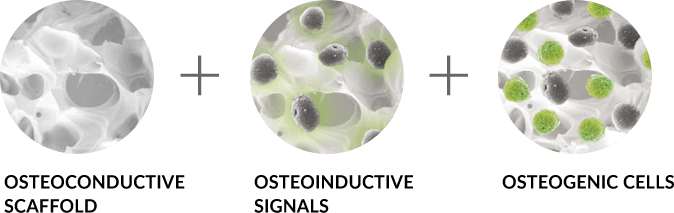
Basic science and preclinical studies have shown that the ideal graft provides three essential components for bone healing. These components are: a scaffold for osteoconduction, signals in the form of growth factor(s) for osteoinduction, and cells from the host or a donor for osteogenesis. There are a variety of biologic solutions available to spine surgeons that provide some or all of these essential components. However, the formulation and quality of the material, how it is delivered and secured to the surgical site, whether it must be mixed with excess exogenous activators (i.e. thrombin), and whether harsh chemicals are used during the production process (i.e. hydrogen peroxide) can all have an effect on the quality of bone healing.
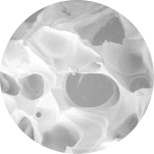
Unlike other commercially available systems, the Fibrinet System’s patented process concentrates platelets into a robust fibrin scaffold, allowing physicians to deliver the platelets and associated growth factors into a defect site without excess exogenous activators (i.e. bovine thrombin). The resulting Platelet-Rich Fibrin Membrane (PRFM) has enhanced mechanical properties with respect to all other similar systems described in the literature.8 Studies have shown that the modulus and tensile strength of PRFM was 600x the stiffness of conventional fibrin clots, it had comparable mechanical properties to arterial tissue, and was approximately 50% the stiffness of human skin.8
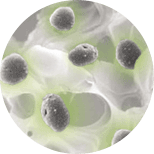
The ability to deliver concentrated platelets without thrombin has demonstrated chondrogenesis and osteogenesis in an animal model whereas concentrated platelets activated with thrombin inhibited such events.6 Scanning electron microscopy images showed intact platelets on the fibrin construct and 99.1% of the platelets were present.8 Other authors have evaluated the percent of viable platelets in the same PRFM structure and demonstrated greater than 50% viability out to seven days.9 A separate animal study evaluating the PRFM in an extraction socket model demonstrated rapid bone healing compared to Demineralized Freezedried Allograft alone.10 These results support the work from Han et al., which showed that concentrated platelets in a boney defect without thrombin can effect bone healing in an animal model.
Autologous platelets contain a variety of associated growth factors (signals) that play different roles in the healing process. As these growth factors have a short half-life, the sustained availability of platelets during the healing phase is very important.11 In three separate characterization studies, associated growth factors from platelets contained in the PRFM were analyzed in culture. Sen et al. evaluated PDGF-AB, VEGF and TGF-B from the PRFM and the results showed increased concentrations out to seven days. Below is a list of known qualities from the literature of the growth factors tested.
The sustained availability of concentrated platelets and their associated growth factors in the PRFM is well documented. In vitro studies have also demonstrated the PRFM’s ability to proliferate cells in culture. A potential source of cells can be found in cancellous bone elements from the patient. With the Fibrinet System, surgeons have the ability to improve the handling characteristics of the bone graft by mixing concentrated platelets with autologous cancellous bone elements and bone grafting material, and delivering them to the bone graft surgical site.
Images courtesy of Herbert Dardik, MD (HD) and Steven Arnoczky, DVM (SA)
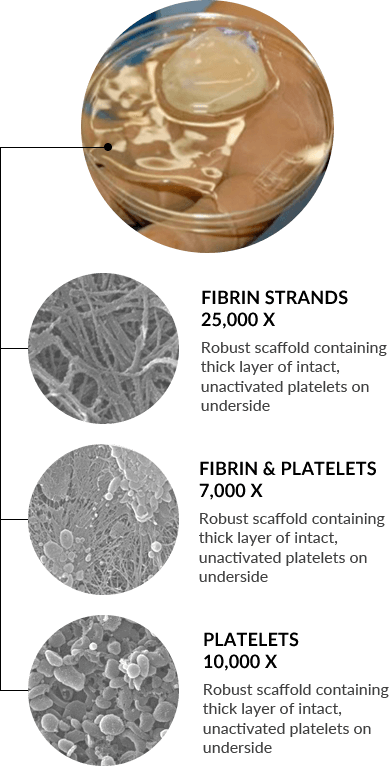
Scanning Electron Micrograph (SEM) image shows a robust, fibrin scaffold with clusters of platelets in the PRFM (HD)
SEM image shows clusters of intact, viable platelets in the PRFM (HD)
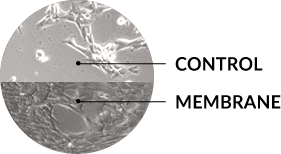
In-vitro, eluent from the membrane consistently induced a significant increase in cell proliferation compared to a standard blood clot at each time point (SA)

1. Sengupta DK, Truumees E, Patel CK. Outcome of Local Bone Versus Autogenous Iliac Crest Bone Graft in the Instrumented Posterolateral Fusion of the Lumbar Spine. Spine 2006; Vol. 31; 9:985-991.
2. Niu CC, Tsai TT, Fu TS. A Comparison of Posterolateral Lumbar Fusion Comparing Autograft, Autogenous Laminectomy Bone With Bone Marrow Aspirate, and Calcium Sulphate With Bone Marrow Aspirate. Spine 2009; Vol. 34; 25:2715-2719.
3. Brenner R, Zhang K, Abjornson C, Cammisa FP. Platelet Rich Fibrin Matrix in Posterolateral Lumbar Fusion. AAOS 2012.
4. Brecevich A.T., Kiely P., Abjornson C, Cammisa FP. A Retrospective Analysis of Platelet Rich Fibrin Matrix Use in Posterolateral Spine Arthrodesis. ISASS 2014.
5. Park JJ, Hershman SH, Kim YH. Updates in the Use of Bone Grafts in the Lumbar Spine. Bull Hosp Jt Dis. 2013; 71(1):39-48
6. Han B, Woodell-May J, Ponticiello M, Yang Z, Nimni M. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoconductivity. J Bone Joint Surg. 2009; 91:1459-70.
7. DePaula CA, Truncale KG, Gertzman AA, Sunwoo MH, Dunn MG. Effects of hydrogen peroxide cleaning procedures on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank. 2005;6:287–298.
8. Lucarelli E, et al. A recently developed bifacial platelet-rich fibrin matrix. Eur Cells Materials 2010; 20:13-23.
9. Roy S, Sen CK, et al. Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Rep Regen. 2011;19(6):753-766.
10. Socket Healing Following the Use of Autologous Platelet-Rich Fibrin Matrix (PRFM) to Ridge Preservation Procedures Employing Demineralized Freeze Dried Bone Allograft Material and Membrane, The Open Dentistry Journal 2009; Simon BI, Zatcoff AL, Kong JJW and O’Connell SM; Clinical and Histologic Comparison of Extraction.
11. Marx RE: Platelet-rich plasma (PRP): What is PRP and What is not PRP? Implants Dental 10:225-228, 2001.
12. Visser LC, Arnoczky SP, Caballero O, et al. Platelet-rich fibrin constructs elute higher concentrations of TGF-ß1 and increase tendon cell proliferation over time when compared to blood clots of similar volume: A comparative in vitro analysis. Vet Surg. 2010c; 39(7):811-817.
13. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial PRP separation systems. Am J Sports Med. ePub Nov 4, 2010.