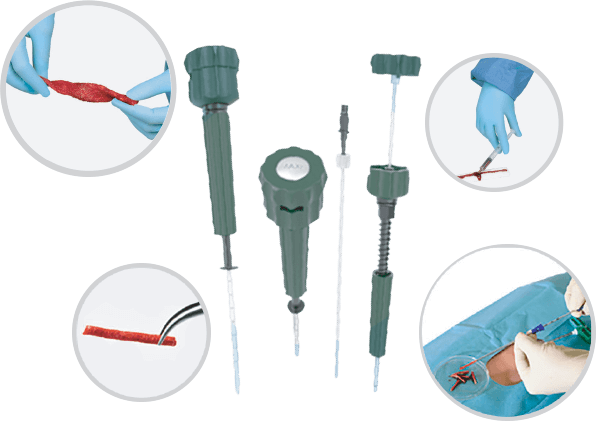
Advanced CellTM is a new advanced bone grafting and fusion convenience kit that offers multiple solutions to achieve optimal results in a variety of settings. Advanced CellTM offers 3 unique technologies all in one convenience kit that have the ability to enhance clinical outcomes.
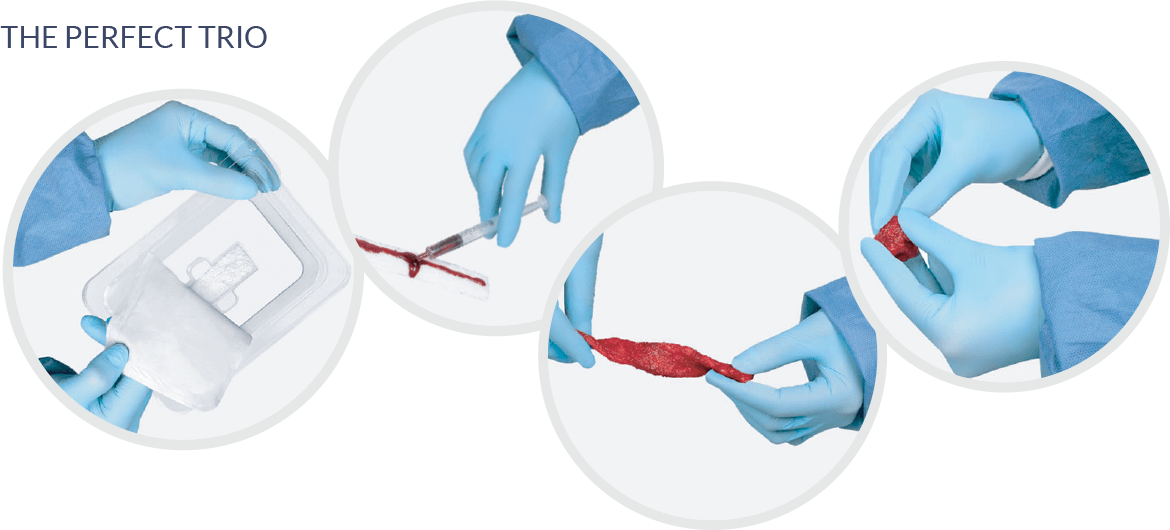
Advanced CellTM offers the ability to harness the power of each patients own live cells and combine with autograft and advanced bone grafting solutions, point of care. Advanced CellTM streamlines OR time and optimizes workflow all while providing low costs solutions for providers.

Maxx-Cell® has created a new gold standard in bone marrow aspiration therapy for various uses in orthopedics, sports medicine and pain management. Deliver the highest, most pure enriched form of bone marrow aspirate without the need for centrifugation. Maximize cell count yields and CFU’s while minimizing peripheral blood dilution. “Maxx-Cell® uses its technology to harvest high quality stem and progenitor cells from various levels within the marrow space, limiting peripheral blood contamination.”
Aspirating Bone Marrow from multiple locations allows for the maximum amount of progenitor stem cells are collected, without the dilution or collection of peripheral blood.

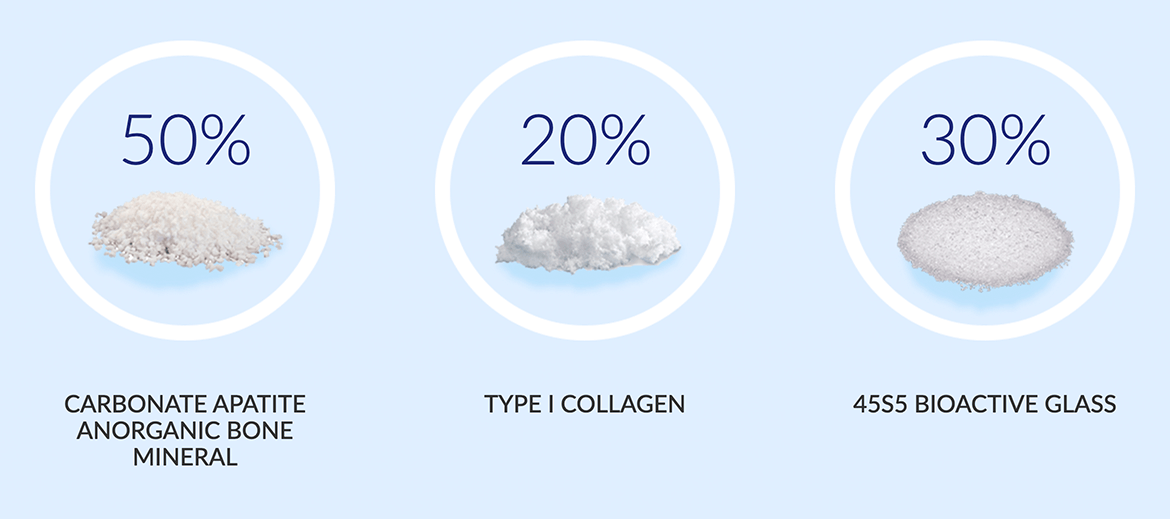
Indications For Use:
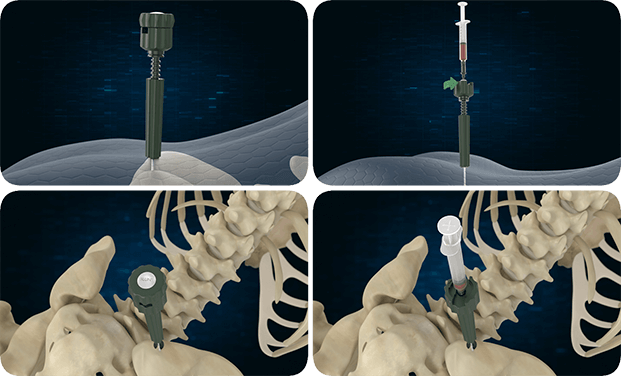
Combined with either autogenous bone marrow or autograft with saline is indicated for bony voids or gaps, that are not intrinsic to the stability of the bony structure. Bio-Reign™ Bioactive Moldable can also be used with autograft as a bone graft extender.
The device is to be gently packed into bony voids or gaps of the skeletal system (i.e., posterolateral spine). These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone. The device resorbs and is replaced with bone during the natural healing process.
8 gauge bone marrow biopsy needle used for percutaneous trephine extraction of autogenous bone marrow/cancellous bone dowels.
Bone marrow aspiration and trephine extraction of cancellous bone are considered minimally invasive techniques compared to open methods of bone extraction and has been documented to have less donor site morbidity than open procedures.(1,2,3).
Research demonstrates the enhanced survival of a bone graft as long as its primary blood supply is preserved. A living bone graft will shorten the time for bony onion because the reconstructed bone is comparable to a bone with a double fracture. (6,7)
Allogeneic or synthetic bone chips hydrated with marrow can be packed around the living bone graft/core to accelerate anastomosis into the graft and minimize morbidity. (6,7)
