Amnio-Maxx is a family of amnion patch allografts that may be used as an anatomical barrier in numerous clinical applications. The natural properties of amniotic tissue help provide mechanical protection to damaged tissue, while the Amnio-Maxx proprietary process retains nutrient-rich growth factors essential for signaling.

Amniotic membrane is a semi-transparent and resilient membrane that lines the upper cavity of the placenta
Amniotic tissue acts as an immune-privileged protective barrier during fetal development1
Amnio-Maxx is applied as an anatomical barrier that helps provide mechanical protection while retaining endogenous growth factors1,2,4
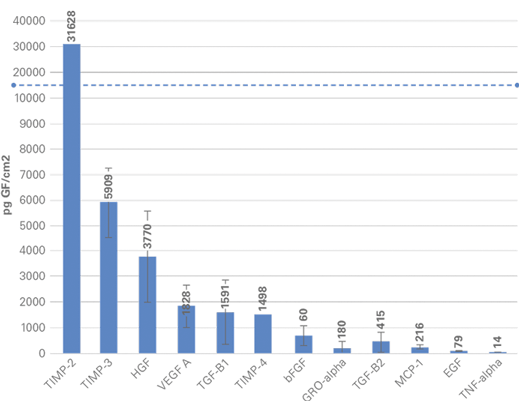
The Amnio-Maxx proprietary process preserves the natural properties of amniotic tissue, maintaining inherent levels of key extracellular matrix molecules, including proteins, carbohydrates, growth factors, and cytokines5


Proper orientation for Amnio-Maxx is achieved when the epithelial layer is facing upwards and the stromal layer is facing downwards, as indicated by:
1. a triangular notch positioned in the upper left hand corner of the allograft (as shown below)
2. An orientation indicator sticker located on the inner pouch facing upwards.