
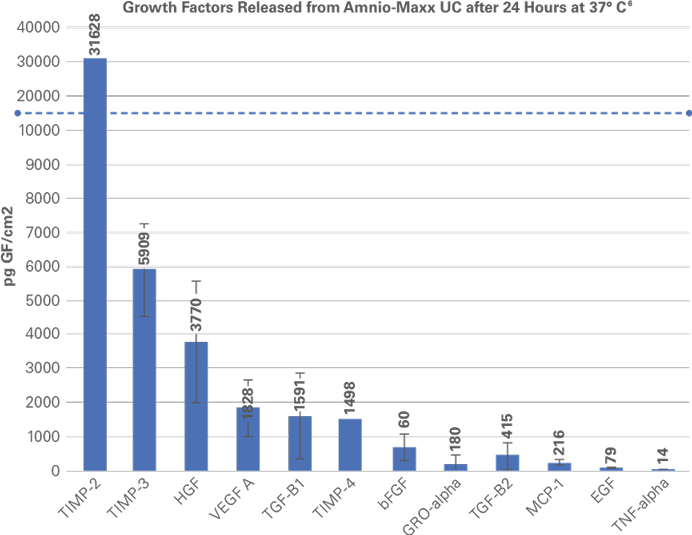
Amnio-Maxx® is a family of amnion patch allografts that may be used as an anatomical barrier in numerous clinical applications. The natural properties of amniotic tissue help provide mechanical protection to damaged tissue, while the Amnio-Maxx® proprietary process retains nutrient-rich growth factors essential for signaling.1,2

| Amnio-Maxx® DL QCODE 4239 | |
|---|---|
| SIZE | CODE |
| 2x3cm | AMN-23 |
| 4x4cm | AMN-44 |
| 4x6cm | AMN-46 |
| 4x8cm | AMN-48 |
| Amnio-Maxx® SL QCODE 4239 | |
|---|---|
| SIZE | CODE |
| 2x3cm | AML-23 |
| 3x3cm | AML-33 |
| 4x4cm | AML-44 |
| 4x6cm | AML-46 |
| 4x8cm | AML-48 |
| 7.5x13cm | AML-713 |
| 10x15cm | AML-1015 |